D.L. Kolesnik*, O.N. Pyaskovskaya, O.V. Yurchenko, G.I. Solyanik
R.E. Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology, NAS of Ukraine, Kyiv 03022, Ukraine
Submitted: May 06, 2019.
*Correspondence: E-mail: [email protected] Abbreviations used: DCA — sodium dichloroacetate; MTF — metformin; Δψm — mitochondrial membrane potential; IC50 — half maximal inhibitory concentration
Abstract
It is known that the arsenal of chemotherapeutic agents for the treatment of malignant brain tumors is quite limited, which causes the high relevance of research aimed at finding new effective antitumor regimens, including the use of energy metabolism modifiers. Aim: To investigate the anti-glioma activity of sodium dichloroacetate (DCA) and metformin (MTF) used in combination in vitro and in vivo. Materials and Methods: Cell survival, cell cycle, apoptosis, mitochondrial membrane potential (Δψm), ATP level, the glucose consumption rate, and lactate production rate were determined in vitro in cultured glioma C6 cells. The antitumor action of agents in vivo was evaluated routinely by the prolongation of the life span of rats with transplanted intracerebral glioma C6 and was confirmed by histological examination of tumor tissue. Results: The half maximal inhibitory concentration (IC50) for DCA and MTF used separately was 79.2 ± 2.1 mM and 78.4 ± 4.0 mM, respectively, whereas IC50 for DCA used in combination with 7.8 mM MTF was 3.3 fold lower (24.0 ± 1.2 mM, p < 0.05). The 1-day incubation of cells with DCA at a concentration close to IC50 (25 mM), in combination with MTF at a concentration by order lower than IC50 (7.8 mM), in contrast to their separate use, resulted in a decrease in the number of viable cells by 40% (p < 0.05); redistribution of the cells by the cell cycle phases toward decreased proportion of cells in the S-phase by 46% (p < 0.05) and an increased percentage of cells in the G0/G1 phase by 24% (p < 0.05) compared to similar indices in the control. High proapoptotic activity of DCA in combination with MTF was supported by a significantly higher percentage of apoptotic cells in vitro than in the control (18.9 ± 4.4% vs 5.7 ± 1.3%, p < 0.05) and a high number of tumor cells with signs of apoptosis revealed during the histological examination of tumor pathomorphosis. The combined effect of DCA and MTF resulted in almost 4-fold decrease of the glucose consumption rate by glioma C6 cells (0.23 ± 0.05 μmol/106 cells/h vs 0.91 ± 0.12 μmol/106 cells/h, p<0.05) compared to the corresponding parameters in the control, and 2-fold increased rate of lactate production (1.06 ± 0.03 μmol/106 cells/h vs 0.53 ± 0.03 μmol/106 cells/h, p < 0.05). At the same time, both Δψm and the level of intracellular ATP in the glioma C6 cells treated with DCA and MTF, both separately and in combination, did not differ significantly from those indices in the control. In in vivo studies, the average life span of rats with intracranial transplanted glioma C6, treated with DCA in combination with MTF in a total dose of 1.1 and 2.6 g/kg body weight, respectively, was 50% higher (p < 0.001) than in the control group. In contrast, in the case of single-use (at a dose of 2.6 g/kg), MTF increased the life span of tumor-bearing animals just by 19% (p < 0.01), whereas DCA alone (at a dose of 1.1 g/kg) did not significantly change the survival time of rats. Conclusions: The obtained data indicate synergism of anti-glioma action of DCA and MTF in a case of their combined use both in vitro and in vivo and may be considered a starting point for the development of effective treatment regimens for malignant brain tumors based on the combined use of DCA and MTF.
Keywords: glioma C6, sodium dichloroacetate, metformin.
Malignant gliomas are known to be the most aggressive human tumors usually with unfavorable prognosis, even with the current advances in antitumor therapy, including anti-angiogenic therapy, targeted molecular therapy, and immunotherapy. The introduction of new methods of multimodal anti-glioma therapy leads to some increase in the average life expectancy of patients, but, unfortunately, usually a patient gains no more than 3–6 months of life [1–4]. Taking into account the undeniable renaissance in the field of studies related to glucose metabolism in tumor cells [5, 6], on the one hand, and the high dependence of brain cells on glucose [7], on the other hand, attempt to increase the effectiveness of antitumor therapy by modifying metabolic pathways of glucose utilization in tumor cells seems to be promising.
In experimental studies metformin (MTF), an inhibitor of the mitochondrial respiratory chain (complex I), which is widely used to treat type 2 diabetes as a hypoglycemic agent, has shown the ability to inhibit proliferation and migration of glioblastoma cells [8], to inhibit the growth of glioma U87 and LN18 xenografts in animals [9] etc. The results of an epidemiological study using a large validated database showed that the long-term use of MTF, although not associated with a reduction in the risk of glioma development, however, contributes to better survival of patients with malignant glioma [10].
The antitumor activity of sodium dichloroacetate (DCA), a non-cancer drug that has been used for 30 years as a means for the treatment of metabolic disorders, in particular, lactic acidosis, has been confirmed in vitro and in vivo, in particular, against glioma cells [11–13].
The aim of this work was to investigate the antiglioma activity of DCA and MTF in the case of their combined use in vitro and in vivo.
MATERIALS AND METHODS
Experimental animals and tumor cell lines. The studies were carried out on inbred female rats 2.5–3 months old weighing 100–130 g from the vivarium of the R.E. Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology (IEPOR) of the National Academy of Sciences of Ukraine (NASU), Kyiv, Ukraine. The research on animals was carried out in accordance with the provisions of the General Ethical Principles of Animal Experiments adopted by the First Congress on Bioethics (Kyiv, 2001) and international requirements in accordance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Purposes (Strasbourg, 1986).
As an experimental tumor model, the glioma C6 cell line was used, which was obtained from the National Bank of Cell Lines and Tumor Strains of IEPOR NASU.
The glioma C6 cells were maintained in vitro in DMEM with 10% fetal calf serum (Sigma, USA) and 40 mg/ml gentamicin at 37 °С in a humidified atmosphere with 5% СО2.
For in vivo experiments, the cells were grown in vitro under standard conditions and transplanted to rats under general anesthesia by intracerebral inoculation of 0.6•106 cells in 0.05 ml of physiological saline into the left parietal region of the brain.
Agents, doses and administration mode. DCA (Sigma, USA) and MTF (Sigma, USA) were used as compounds under study.
To evaluate the antitumor activity of DCA, MTF and their combination in rats after tumor cell inoculation, the animals were randomized by weight and distributed into 4 groups: 1)rats administered with DCA only in a total dose of 1.1 g/kg (n = 12); 2)rats administered with MTF only in a total dose of 2.6 g/kg (n = 12); 3)rats administered with DCA in combination with MTF (n = 13) at the above doses; and 4) rats administered with water (control, n = 12).
In all cases, the agents were dissolved in water and administered orally with a probe, once a day, starting from the first day after the inoculation of tumor cells. In the case of the combined use of DCA and MTF, the latter was administered 3–4 h after the administration of DCA.
All agents were prepared ex tempore.
The cytotoxic/cytostatic effects of DCA and MTF alone and in combination were assessed by the half maximal inhibitory concentration (IC50) index — concentration of the agent, which causes a 50% reduction in the number of viable cells in relation to control due to its cytotoxic and/or cytostatic action.
Glioma C6 cells were seeded in a 35 mm Petri dish at a density of 0.5•106 cells/ml. After the preincubation period (16–18 h), the medium was replaced by a fresh one with the addition of the investigational agents, and incubation continued for 1 day.
In all cases, the cells that were incubated under analogous conditions, but without the addition of any agents, served as control.
Each agent concentration and combination of these agents were evaluated in 3 replicates (for IC50 determination) or 5 replicates (for determining effects of 25 mM DCA, 7.8 mM MTF, and their combination).
The number of viable cells at the beginning of incubation (N0) and after 1 day (N24) was evaluated by their direct counting in a hemocytometer using trypan blue.
The distribution of cells by the cell cycle phases and the level of apoptosis were assessed by flow cytometry using propidium iodide and RNAase A [14]. The number of apoptotic cells was evaluated by the sub-G0/G1 peak.
The rate of glucose consumption and the rate of lactate production by tumor cells was estimated based on the contents of the substrates in the incubation medium and the number of viable cells at the beginning of the incubation period and after 1 day.
The level of glucose and lactate was determined in deproteinized samples of supernatants using a biochemical analyzer and commercial kits for their determination.
The internal mitochondria membrane potential (Δψm) of tumor cells was determined using flow cytometry and the cationic dye JC-10 (Sigma, USA) by the ratio of fluorescence intensity in the red (FL2) and green (FL1) regions.
The effectiveness of the antitumor action of the investigated agents was assessed by life span prolongation (LSP) of animals in experimental groups compared with the life span of animals in the control group, which was calculated by the formula:
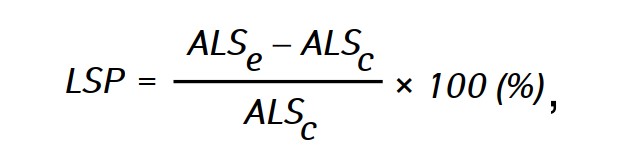
where ALSe and ALSc— the average life span of rats in experimental and control groups respectively.
For histological examination, tumor tissue samples of the rats with glioma C6 were fixed in 10% neutral buffered formalin solution for 24 h, dehydrated in alcohols of ascending concentrations and embedded into paraffin. Histologic sections were stained with hematoxylin-eosin and analyzed using the AxioStarPlusZeiss microscope.
Statistical analysis of the data was performed using descriptive statistics, Student’s t-test and Mann— Whitney u-test, nonlinear regression analysis using Microsoft Excel and Microcal Origin software. Data are presented as M ± m, where M is the mean value; m is the standard error of the mean value.
RESULTS AND DISCUSSION
We have shown that MTF significantly enhanced the cytotoxic and/or cytostatic activity of DCA against glioma C6 cells in vitro. In particular, in the case of its single-use, IC50 of DCA was 79.2 ± 2.1 mM, while this index for DCA used in combination with MTF even in low non-cytotoxic concentration decreased more than three times (p < 0.05) (Table 1, Fig. 1). In addition, if necrotic cell death due to the action of DCA and MTF used separately was fairly low in a wide range of concentrations of both agents and did not exceed 15% even at the maximum investigated concentrations, the use of DCA in combination with 7.8 mM MTF resulted in a sharp concentration-dependent increase in the number of dead cells, especially in the range of concentrations of DCA higher than IC50 (Fig. 1, c).
| Agent | DCA | MTF | IC50, mM |
| DCA | 10–100 | – | 79.2 ± 2.1 |
| MTF | – | 7.8–80 | 78.4 ± 4.0 |
| DCA + MTF | 10–40 | 7.8 | 24.0 ± 1.2* |
Note: *p < 0.05 as compared to the corresponding value in the case of single-use.
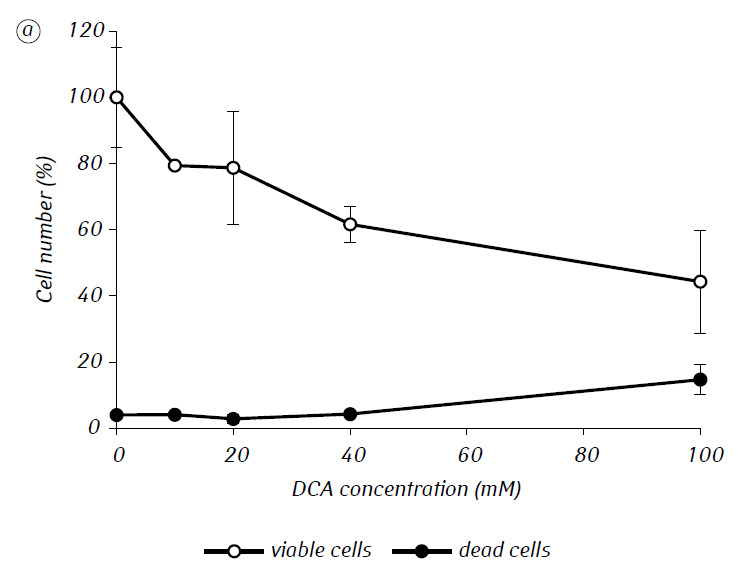
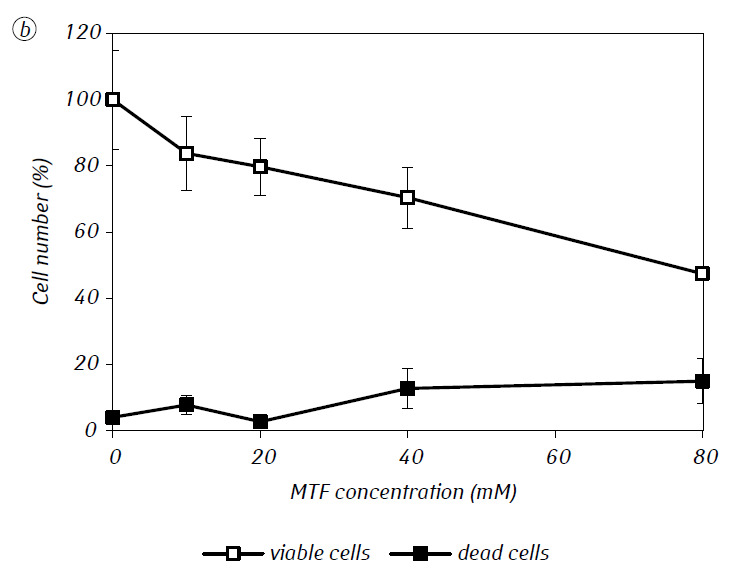
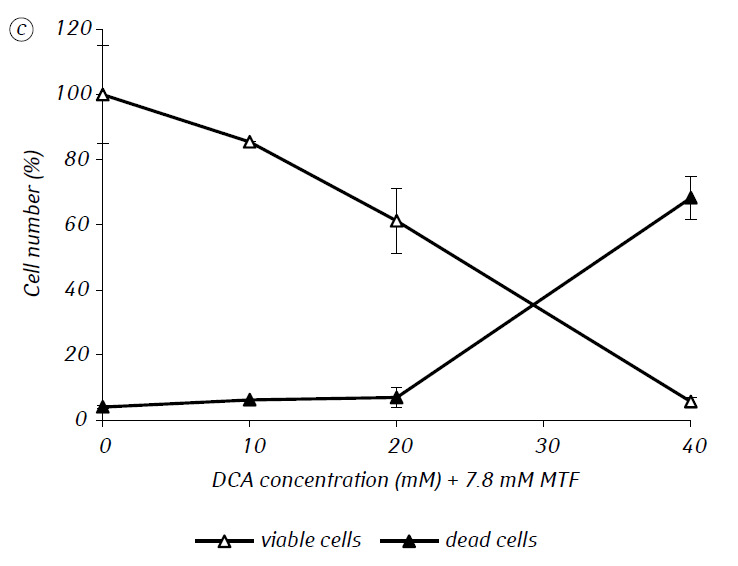
For a more detailed assessment of the synergistic action of the two agents, the glioma C6 cells were incubated for 1 day with 25 mM DCA (concentration close to IC50) and 7.8 mM MTF (concentration less than IC50), in combination and separately. The obtained data on their influence on the growth characteristics of glioma C6 cells, as well as the characteristics associated with their energy metabolism, are presented in Table 2.
| Index | Control | 25 mM DCA | 7.8 mM MTF | 25 mM DCA+ 7.8 mM MTF |
| Number of viable cells, % | 100.0 ± 5.3 | 91.5 ± 2.3 | 85.3 ± 6.7 | 60.2 ± 2.0 |
| N24/N0 | 1.9 ± 0.1 | 1.8 ± 0.04 | 1.7 ± 0.1 | 1.2 ± 0.04* |
| Necrosis, % | 2.6 ± 0.6 | 2.9 ± 0.8 | 5.5 ± 1.6 | 6.9 ± 2.6 |
| Apoptosis, % | 5.7 ± 1.3 | 30.2 ± 4.1* | 5.6 ± 2.5 | 18.9 ± 4.4* |
| G0/G1, % | 49.0 ± 2.3 | 49.6 ± 2.2 | 46.2 ± 3.3 | 60.8 ± 1.3* |
| G2/M, % | 30.7 ± 4.3 | 27.5 ± 1.7 | 29.7 ± 2.8 | 28.2 ± 3.07 |
| S, % | 20.3 ± 2.7 | 22.9 ± 1.0 | 21.1 ± 3.3 | 11.0 ± 1.8* |
| Δψm (FL2/FL1) | 0.85 ± 0.15 | 0.97 ± 0.01 | 0.81 ± 0.04 | 0.96 ± 0.01 |
| The glucose consumption rate, µmol/106 cells•h | 0.91 ± 0.12 | 0.16 ± 0.02* | 0.46 ± 0.07* | 0.23 ± 0.05* |
| The lactate production rate, µmol/106 cells•h | 0.53 ± 0.03 | 0.42 ± 0.01* | 1.1 ± 0.02* | 1.06 ± 0.03* |
| ATP level, pmol/106 cells | 9835 ± 357 | 9819 ± 292 | – | 9379 ± 191 |
Note: *p < 0.05 as compared to corresponding index in control.
As can be seen from Table 2, the synergistic action of DCA in combination with MTF against glioma C6 cells was confirmed by a sharp decrease in the number of viable cells by almost 40% (p < 0.05) compared to the corresponding index in the control, whereas in the case of their independent use the number of viable cells was not affected significantly. At the same time, the number of dead cells in all cases was low and practically did not differ from that in the control.
Similarly, no significant effect of DCA in combination with MTF on the induction of apoptosis in glioma C6 cells was detected. The sharp increase of more than three times (p < 0.05) of the number of apoptotic cells in the case of combined use of DCA and MTF occurred only due to the action of the DCA itself. The latter was confirmed by the high level of DCA-induced apoptosis, which in the case of its separate use by 5.3 times (p < 0.05) exceeded the corresponding value in the control (Table 2).
In contrast to DCA, 7.8 mM MTF used alone did not increase apoptosis in these cells and even reduced the sensitivity of cells to the proapoptotic action of DCA if used in combination. In particular, the number of apoptotic cells induced by the combination of DCA and MTF was almost 1.5 times lower (p < 0.05) than DCA-induced one.
The synergistic anti-glioma effect of DCA and MTF was also confirmed by almost 24% increase (p <0.05) of percentage of cells in the G0/G1 phase and a significant decrease in their fraction (by almost 46%, p < 0.05) in the S phase, whereas DCA and MTF used separately did not affect significantly the cell cycle phase distribution (Table 2).
Attention is drawn to the fact that the addition of DCA and MTF, both separately and in combination, significantly reduced the rate of glucose consumption. In contrast, the rate of lactate production in cells treated with DCA was sharply reduced, while this index in cells treated with MTF, alone or in the combination, increased significantly.
In particular, in the cells treated with DCA, there was observed a sharp drop in glucose consumption rate by 82% (p < 0.05) along with 21% (p < 0.05) decrease in the rate of lactate production compared with similar control parameters. The MTF, unlike DCA, greatly increased the production of lactate by tumor cells, both in case of a separate use or in combination with DCA. The rate of lactate production increased by almost 108% (p < 0.05) and by 100% (p < 0.05) in cells treated with MTF alone and in combination with DCA, respectively, compared with that in the control. Interestingly, despite the significant effect of DCA and MTF on tumor cell metabolism they practically did not reduce the level of ATP in tumor cells. As can be seen from Table 2, the content of intracellular ATP in cells treated with DCA and MTF alone, or in combination, did not differ significantly from that in the control.
In in vivo experiments, MTF significantly enhanced the antitumor effect of DCA, resulting in the synergy of their action. The survival curves of experimental animals are presented in Fig. 2. The average life span of rats with intracranial transplanted glioma C6 treated with DCA only, practically did not differ from that in control, whereas the administration of MTF only significantly prolonged the life span of rats with tumors by almost 20% (p < 0.01). Combined use of DCA and MTF resulted in a significant increase in their anti-glioma effects, which was confirmed by a significant prolongation of life span of animals by 50% (p < 0.001) and by more than 26% (p < 0.05) compared to this index in the control group and in the group of rats, which were administered MTF only, respectively.
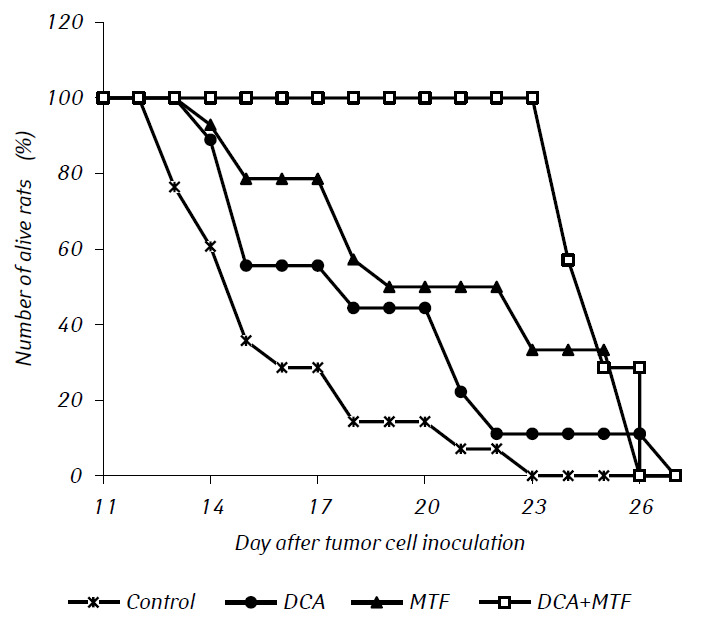
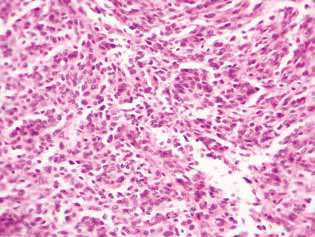
Histological examination of tumor tissue of rats with glioma C6 revealed some changes in its structure, which correlated with the high efficiency of the antiglioma action of the investigated agents. In control animals, the tumor usually developed in the molecular or lower layers of the cerebral cortex. Morphologically, the zone of neoplasia was represented by the accumulation of large cells and nuclei of a diverse form, hyperchromic or with moderate chromatin content. In the samples studied, cellular polymorphism, and the presence of multi-nucleus large cells were typical. Tumor cells possess, as a rule, elongated shape with a cytoplasm, with fibrous structures, and wellexpressed nuclei. Some cells were smaller in size, rounded, with dense hyperchromic nuclei (Fig. 3).
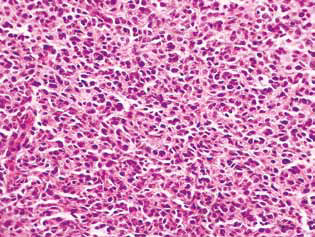
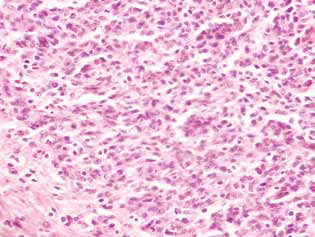
In rats treated with DCA and MTF alone or in combination, in the tumor tissue, there was observed an accumulation of apoptotic cells or cells with hyperchromic ring-shaped structures at the nuclei periphery as evidence of activation of apoptosis. It should be noted that these phenomena were much more expressed in the case of the combined use of the agents (Fig. 4).
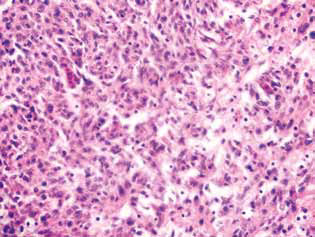
A marked manifestation of the anti-glioma action of DCA in combination with MTF was a significant decrease in satellite regions in the brain tissue (Fig. 5). In the control group of animals, the satellite structures were located near the main node of the tumor and were fairly common. Around the several malignant cells that migrated from the main tumor tissue, obviously, the process of neoplastic angiogenesis was launched, which led to the formation of a blood vessel and the formation of a multilayered rim from the malignant cells around it, which was then accompanied by the merging of such structures with the main lesion and an increase in the tumor size (Fig. 5, a).
Instead, in animals receiving DCA in combination with MTF, a sharp decrease in the formation of satellite tumor lesions was observed, which may be due to the suppression of neoangiogenesis and the inability to form new sites of neoplastic growth (Fig. 5, b). In addition, the tumor pathomorphosis in animals receiving DCA in combination with MTF was manifested by the presence of clear devastated tumor lesions (Fig. 6) and a sufficiently large number of destroyed and devastated blood vessels in the tumor tissue (Fig. 7).
Consequently, significant inhibition of glioma C6 cell proliferation in vitro due to the combined use of 25 mM DCA and 7.8 mM MTF, which contrasted with minor cell death, was mainly due to their cytostatic action. The cytostatic component in the synergistic effect of DCA and MTF in relation to glioma C6 cells was also confirmed by the significant accumulation of cells in the G0/G1 phase and the decrease in their fraction in the S-phase.
Apparently, the enhancement of the anti-glioma action of DCA and MTF due to their combination was caused by their multi-directional effects on the energy metabolism of tumor cells. In particular, the rate of lactate production in glioma C6 cells without the addition of any agents (control) was almost twice lower than the rate of glucose consumption. Such a correlation between these indices indicated the occurrence in these cells of not only the glycolytic conversion of glucose to lactate but also the rather high activity of oxidative phosphorylation and anabolic processes of synthesis of biomolecules.
In DCA-treated cells, a sharp decrease in glucose consumption along with a simultaneous decrease in the rate of lactate production indicated that DCA at 25 mM concentration was able to realize its potential as a kinase pyruvate dehydrogenase inhibitor and to increase the intensity of mitochondrial respiration and indirectly suppress glycolysis in these cells.
A sharp decrease in the rate of glucose consumption in glioma cells due to the action DCA was associated with its pronounced apoptosis-inducing activity. In spite of the significant pro-apoptotic effect of DCA and the shift of cellular metabolism towards mitochondrial respiration, no apparent changes in the membrane potential of the internal membrane of mitochondria in glioma C6 cells were detected.
Unlike DCA, in tumor cells treated with MTF, there was detected a significant increase in lactate production, both in the case of its separate use or in combination with DCA. Obviously, this happened via inhibition of complex I of the electron transport chain of mitochondria by MTF [15], even in low non-toxic concentrations. Taking into account the fact that glycolysis activation usually promotes the acquisition of tumor cells resistance against pro-apoptotic stimuli [16], more than 1.5 times fall in DCA-induced apoptosis under combined use of the agents can be attributed, at least in part, to MTF-induced activation of aerobic glycolysis in glioma C6 cells.
In the case of the DCA and MTF combination, the MTF-induced modification of energy metabolism was implemented at a greater extent. Given the high rate of lactate production by cells treated with MTF alone or in combination with DCA, it can be concluded that in the latter case, the MTF-induced glycolysis dominated over DCA-induced mitochondrial metabolism in tumor cells.
It should be noted that despite the significant effect on tumor metabolism of DCA and MTF, used separately or in combination, they practically did not reduce the level of ATP in tumor cells. The lack of changes in the ATP level in tumor cells due to the effect of DCA and MTF alone and in combination indicated the implementation of compensatory responses in the cells to maintain cell energy supply and sufficiently high metabolic plasticity of these cells.
Previously, we have shown that the antitumor effect of energy metabolism modifiers, in particular, MTF, substantially depends on the microenvironment of tumor cells and may radically differ from the in vitro effects [17]. In these studies, the synergism of the anti-glioma activity of DCA in combination with MTF was detected not only in vitro but also in vivo.
In in vivo conditions, the high anti-tumor efficacy of the combination of DCA and MTF resulted in a significant prolongation of the average life span of rats with tumors. According to the histological study of tumor tissue of rats with glioma C6, a significant contribution to the anti-glioma action of DCA and MTF has been made by their ability to induce apoptosis if used in combination. Although in vitro there was observed no increase in the pro-apoptotic action of DCA and MTF combination (which apparently was related to a rather low studied concentration of the latter), histological data indicated their ability to synergistically induce apoptosis in the tumors. In the work [18], the synergism of the pro-apoptotic action of these two agents was also shown in relation to ovarian tumor cells.
In addition to pro-apoptotic action, the high efficacy of the anti-glioma effect of DCA in combination with MTF was associated with a decrease of the degree of tumor invasion and the destruction of the blood vessels in the tumor. The latter may cause a deficit of nutrients, in particular, glucose, in the tumor microenvironment resulting in the suppression of tumor growth.
Higher antitumor efficacy of the combination of DCA and MTF may also be due to the involvement of additional mechanisms of agent actions, including their action on normal cells of the body, which can significantly alter the levels of nutrient substrates in the tumor microenvironment, in particular in the blood. This is true for MTF, which, through the activation of AMP-activated protein kinase, is capable of affecting the glucose and lipid metabolism of hepatocytes and skeletal muscles [19], and for DCA, known as universally effective in lowering lactate levels [20]. In particular, we have previously shown that DCA in combination with MTF decreases the levels of lactate and glucose in rat blood, which correlates with their antitumor activity [21]. It is also possible that a certain contribution to the anti-glioma effect of the DCA and MTF combination is that they are able to correct anemia and thrombocytopenia developing against the background of glioma C6 growth in rats [21].
Thus, the presented data indicated the synergism of anti-glioma action of DCA and MTF in the case of their combined use both in vitro and in vivo and can be considered a starting point for the development of effective treatment regimens for malignant brain tumors based on the combined use of DCA and MTF.
REFERENCES
1 1. Taal W, Bromberg JE, van den Bent MJ. Chemotherapy in glioma. CNS Oncol 2015; 4: 179–92.
2 Chowdhary MM, Ene CI, Silbergeld DL. Treatment of gliomas: how did we get here? Surg Neurol Int 2015; 6: S85–8.
3 Wang Y, Xing D, Zhao M, et al. The role of a single angiogenesis inhibitor in the treatment of recurrent glioblastoma multiforme: a meta-analysis and systematic review. PLoS One 2016; 11: e0152170.
4 Ameratunga M, Pavlakis N, Wheeler H, et al. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst Rev 2018; 11: CD008218.
5 Agnihotri S, Zadeh G. Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro-Oncol 2016; 18: 160–72.
6 Weber GF. Time and circumstances: cancer cell metabolism at various stages of disease progression. Front Oncol 2016; 6: 257.
7 Seyfried TN, Sanderson TM, El-Abbadi MM, et al. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer 2003; 89: 1375–82.
8 Seliger C, Meyer A-L, Renner K, et al. Metformin inhibits proliferation and migration of glioblastoma cells independently of TGF-β2. Cell Cycle 2016; 15: 1755–66.
9 Sesen J, Dahan P, Scotland SJ, et al.Metformin inhibits growth of human glioblastoma cells and enhances therapeutic response. PLoS One 2015; 10: e0123721.
10 Seliger C, Renner K. P08.52 Metformin as adjuvant therapy for glioma. Neuro-Oncology 2017; 19: iii65.
11 Michelakis ED, Sutendra G, Dromparis P, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med 2010; 2: 31ra34.
12 Duan Y, Zhao X, Ren W, et al. Antitumor activity of dichloroacetate on C6 glioma cell: in vitro and in vivo evaluation. Onco Targets Ther 2013; 6: 189–98.
13 Fedorchuk AG, Pyaskovskaya ON, Gorbik GV, et al. Effectiveness of sodium dichloroacetate against glioma C6 depends on administration schedule and dosage. Exp Oncol 2016; 38: 80–3.
14 Nicoletti I, Migliorati G, Pagliacci MC, et al. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 1991; 139: 271–80.
15 Wheaton WW, Weinberg SE, Hamanaka RB, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 2014; 3: e02242.
16 Michelakis ЕD, Websterl L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer 2008; 99: 989–94.
17 Pyaskovskaya ON, Kolesnik DL, Fedorchuk AG, et al. Cytotoxic activity of metformin in vitro does not correlate with its antitumor action in vivo. Exp Oncol 2017; 39: 264–8.
18 Bo Li, Xinzhe Li, Zhenhong Ni, et al. Dichloroacetate and metformin synergistically suppress the growth of ovarian cancer cells. Oncotarget 2016; 7: 59458–90.
19 Zhou G,Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–74.
20 Abdelmalak M, Lew A, Ramezani R, et al. Long-term safety of dichloroacetate in congenital lactic acidosis. Mol Genet Metab 2013; 109: 139–43.
21 Prokhorova IV, Pyaskovskaya ON, Kolesnik DL, Solyanik GI. Influence of metformin, sodium dichloroacetate and their combination on the hematological and biochemical blood parameters of rats with glioma C6. Exp Oncol 2018; 40: 205–10.